Hard capsules have traditionally been manufactured from gelatin. Gelatin is obtained from bone or skin (calf or pig) acid or alkali treatment over a period of weeks, in some cases if 30 weeks (pork skin, 1–5% HCl). The product pH is adjusted, and a hot water extraction is accompanied by filtration, concentration, and solidification. The final product is milled to size.
Capsule shells are prepared by dipping manganese bronze pins into a bath of molten gelatin. Once deleted from the bath, the gelatin solidifies on the pins. The caps and bodies are then dried and trimmed. Colorant or titanium dioxide (for opacity) is inserted as part of this process.
The need for capsules with different physicochemical properties, to aid in stability, for example, has promoted a search for alternative materials. In addition, individuals who, for strictly religious or health reasons, cannot ingest gelatin, need alternative products. In this regard, starch and hydroxypropylmethyl cellulose (HPMC) have been developed. There is no reason to think that other film-forming polymers might not be useful in this regard. One significant issue that must be examined is the moisture content of the capsule. Gelatin is known to optimally contain 5% to 15% moisture. Below 5%, the shell becomes brittle and may shatter. Above 15% gelatin distorts, and the shape of dosage form, if not its integrity, is challenged. The presence of a nutrient-rich environment and moisture may offer an ideal situation for microbial growth and enzyme action. Control of microbial growth is, therefore, a fundamental consideration in the preparation of capsule products.
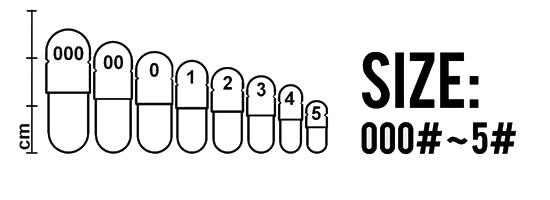
Various capsule sizes are manufactured, as showing in Figure 1. There are not any strict rules for predicting required capsule size. Capsules are chosen on the basis of their capacity and the nature of the formulation to be added. The bulk density and compressibility of the product (drug and excipients) dictate the quantity of drug that can be located within a capsule of known volume. Since the drug dose required to achieve a therapeutic effect can be estimated for different compounds and is known for existing compounds, this information can be used in conjunction with the capsule volume to select an appropriate size.
Requirements for capsule production depend on the scale of manufacturing. Extemporaneous preparation (6–12 capsules) usually employs plenty of the product formula to fill one more capsule than required, to account for loss of fill in manipulation. Special consideration should be given to control substances where all of the drug must be accounted for. Industrially (thousands). The amount necessary to fill the desired number can be prepared because the error will be small on such a large scale. The operations involved in large- or small-scale capsule filling are the same. Capsules as supplied in random orientation must be rectified into a bodies-down, caps-up orientation. The two shells are then separated, and the capsule is filled with the product formulation. Numerous methods are available to fill the capsules. For small-scale production, a plate or single capsule filling method is employed. On a larger scale, tamping, intermittent compression, continuous compression vacuum, or auger filling may be employed (Fig. 2). The shells are then joined and sealed, and the completed product is discharged, as showed in Figure 3. Different locking.
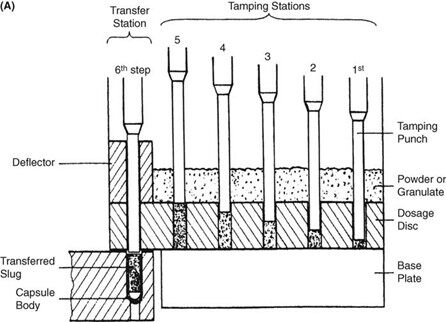
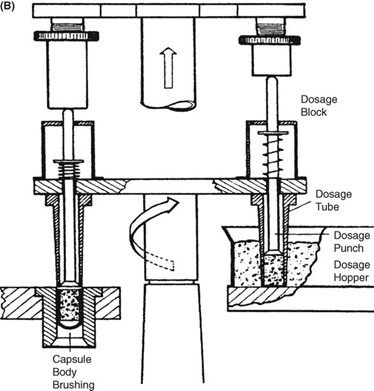
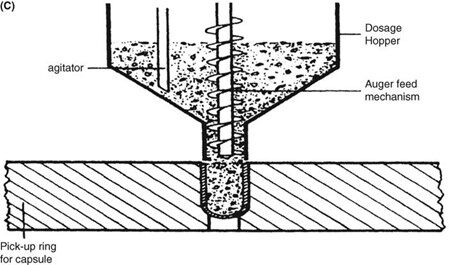
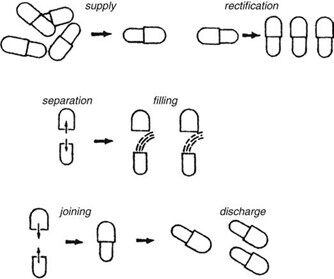
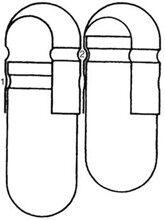
Mechanisms have been developed for capsules shown in Figure 4. Cleaning and polishing step also follows the manufacturing procedure to improve product appearance.
The product is visually inspected following production, its potency and uniformity are evaluated, and it is transferred hygienically to the final Packaging. If the product is hygroscopic, it may be necessary to package capsules with desiccant to avoid moisture uptake. Alternatively, impervious packaging materials, such as aluminum blisters, may be used. Capsules are easy to prepare than tablets, are quite flexible with respect to dose, and are easily combined with other solid dosage forms since other capsules or tablets can be incorporated into larger capsules.